Introduction to Molecular Modeling for K-12 students- A STEM Activity
The following activity is part II of the Introduction to Molecular Modeling for K-12. It is recommended that you complete Part I first.
PART II Crystal Structures:
Can you tell which of the above structures is liquid water and which is ice?.
Ice is a crystal lattice. All crystals have a repeating structure. Can you locate the repeating structure in ice? You will probably have to change the display to wireframe or sticks to make viewing easier. Look for a repeating geometric pattern. Notice that the water structure does not have this repeating pattern..
Ice floats in water because it is less dense. What makes ice less dense if they are both composed of the same type of molecules? Compare the two structures using the spacefill and wireframe displays. Zoom in for a close look as you rotate the molecule.
Question
12: What are some unique properties of ice? _____________
Question
13: Why do you think ice is less dense than water?_____________
____________________________________________________________________________
GRAPHITE |
DIAMOND |
The above structures are made of only 1 type of atom (element).
Question 14: What is the element ?
Question 15: If both diamond and graphite are made of the same atom types, why is graphite slippery and soft while diamond is hard?
Question 16: What makes diamond a crystal structure? With your partner look for the repeating structure found in diamond.
_______________________________________________________________________
Take a look at the two crystal structures below -- salt and calcite.
Question 17: How are salt and calcite different
from diamond or ice?
Question 18: How are salt and calcite similar
in structure to diamond and ice?
Question 19: Would you expect salt
or calcite to be stronger than diamond? Why?
SALT |
CALCITE |
Click on any atom then look at the bottom of your viewer to see the atom type: C=carbon; O=oyxgen; H=hydrogen; Ca = Calcium; and Cl = Chlorine.
Question
20: What elements is salt composed of?
Question 21: What is the
chemical formula for salt?
Question 22: What elements is
calcite composed of?
Question 23: What is the chemical formula for
calcite?
MATHMOL
- Activity 1: Measuring length and distance at the molecular level
- Activity 2: Geometry-of-1-Dimension
- Activity 3: Geometry of 2- Dimensions
- Activity 4: Geometry of 3-Dimensions
- Activity 5: Introduction to Molecular Modeling using Jsmol
- Activity 6: The Geometry of Crystals
- Activity 7: Summary Sheet by Students
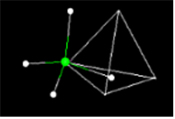
- Activity 8: What is the Geometry of the Methane Molecule
- Activity 9: Geometry of the Crystal Structure of Ice
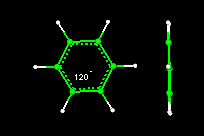
- Activity10:Geometry of the Benzene Molecule